Mon, Nov 3, 2025
[Archive]
Volume 1, Issue 4 (Summer 2023)
CPR 2023, 1(4): 394-409 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yazdani Z, Rafiei A. Imbalance of Regulatory T Cells in Autism Spectrum Disorder: A Review Study. CPR 2023; 1 (4) :394-409
URL: http://cpr.mazums.ac.ir/article-1-59-en.html
URL: http://cpr.mazums.ac.ir/article-1-59-en.html
Department of Immunology, Molecular and Cell Biology Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 2138 kb]
(425 Downloads)
| Abstract (HTML) (1276 Views)
Full-Text: (497 Views)
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by problems in signs of social interactions and communications and repetitive and restrictive behaviors. These behaviors vary in intensity and time of occurrence [1]. Patients with ASD have feeding problems, such as picky eating or food avoidance. They have difficulty falling or staying asleep and have excessive daytime sleepiness. They have even problems in social functioning such as a deficiency in recognizing emotions and interpreting vocal and visual cues [2، 3]. Their families have high levels of anxiety, stress, and depression, and suffer from high financial burden of the disease [4]. According to the World Health Organization (WHO), 1% of the world’s population suffer ASD [5]. It is a major health problem worldwide. Identifying the involved factors in this disease can help prevent and cure it. The biological mechanisms of ASD are unknown, but the existence of some evidence, such as the association of ASD with congenital rubella infection [6، 7], inflammation [8-10], disruption of cytokine regulation [11-15], and autoantibodies against the proteins of the central nervous system [16-19], confirm the role of the immune system dysfunction in ASD. Several reports have shown an association between ASD and a personal or family history of autoimmune disorders such as psoriasis [17، 18], type 1 diabetes [19-21], celiac disease [22، 23], systemic lupus erythematosus [24، 25], rheumatoid arthritis [26، 27], and autoimmune thyroiditis [28]. Other studies using brain tissues or peripheral blood of people with ASD have also reported a dysfunction in the immune system, including the abundance of active M2 microglial genes in the brain and the genes related to the immune responses [20].
Regulatory T cells (Tregs) play an essential role in regulating the function or maintaining the homeostasis in the immune system. The abnormalities in the population of Tregs can Therefore have a role in progress of ASD. Ellul et al. in a meta-analysis of 13 studies assessed Treg lymphocytes/Th17 lymphocytes imbalance in ASD with the participation of 388 ASD patients and 326 healthy controls [21]. There is no narrative review to investigate the effect of imbalance in the number of Tregs and their role in ASD based on molecular mechanism. Therefore, this review study aims to increase knowledge of the relationships between Treg dysfunction and ASD. We first explain the structure and biological function of Tregs in the immune system and identify its relationship with neurodevelopment, and finally investigate its association with the progression of ASD. The search was carried out in online databases including Google Scholar, Web of Science, PubMed, and Scopus for related studies published up to July 2023.
Physiology and function of Tregs
Tregs are a small group of immune cells that prevent autoimmune diseases and control inflammation by inhibiting autoreactive T cells. Therefore, the development of autoimmune diseases or the lack of control over inflammation in other conditions indicates an insufficient number of Tregs [22].Tregs have different origins. They can originate from the thymus during the development of T cells or from naive CD4+ due to /CD28 stimulation in the presence of cytokines such as transforming growth factor-beta (TGF-β) and interleukin-2 (IL-2) in peripheral blood. Moreover, Tregs have different locations. A group circulates in organs and secondary lymphatic fluids, and the other group resides in non-lymphoid tissues such as colon, fat, and skin, and can play roles such as repairing muscles, differentiating oligodendrocytes, and promoting have remyelination in the brain [22، 24]. Forkhead box P3 (FoxP3) and IL-2 receptor alpha chain (CD25) are two markers of these cells. FoxP3 is a crucial regulator of Treg development and function. The transfer of this marker to naive T cells increases the expression of CD25 and other Treg-related cell surface molecules, cytotoxic T cell-associated antigen-4 (CTLA-4), and glucocorticoid-induced TNF receptor family-related gene/protein (GITR). It suppresses the expression of IL-2, interferon‐gamma (IFN-γ), and interleukin-4. Some studies have shown that high FoxP3 expression can confer suppressive activity to normal non-Treg cells. Other studies have shown that Tregs acquire the properties of effector T cells upon the loss of FoxP3 expression [23، 24]. CD25 is also functionally necessary for Treg development. In vitro studies proved that IL-2 is required for the stable expression of FoxP3 and CD25 in Tregs and increasing their suppressive function [25]. IL-2 also induces the differentiation of Tregs.
Suppression mechanisms of Tregs
Tregs can control immune system by suppression mechanism. These cells interact strongly with dendritic cells and suppress conventional naive T cells [26]. Also, they can regulate CD4+T cells. They can control Th1 response by the expression of miR-146a, and suppression of expression and activation of signal transducer and activator transcription 1 (STAT1) [27]. Also, they can inhibit the proliferation of TH1 cells and the production of their cytokines by expression of interleukin-10 (IL-10) and TGF-β [28-30]. Gut microbiota induces a distinct Treg population that expresses Rorγ, and promotes T helper 17 cell differentiation in colonic TH1/TH17 inflammation [31]. Treg can induce apoptosis in CD4+T cells by expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/death receptor 5 (DR5) [32].These cells can suppress CD8+ T cell proliferation by expression of interleukin-35 [33، 34]. Also, they can upregulate the expression of CD95 and CD95L and express granzyme B and perforin to induce apoptosis in these cells [35-38]. In visceral adipose tissue, Tregs catabolize prostaglandin E2 (PGE2) into the 15-keto PGE2 and suppresses conventional T-cell activation and proliferation [39]. Tregs suppress autoreactive B cells through programmed death ligand 1/programmed cell death protein 1 (PDL1/PD-1) interaction [40]. These cells can convert monocytes to a tolerogenic phenotype (M2 Macrophages) and reduce inflammation [41]. Figure 1 shows some mechanisms by which Tregs control immune cells.
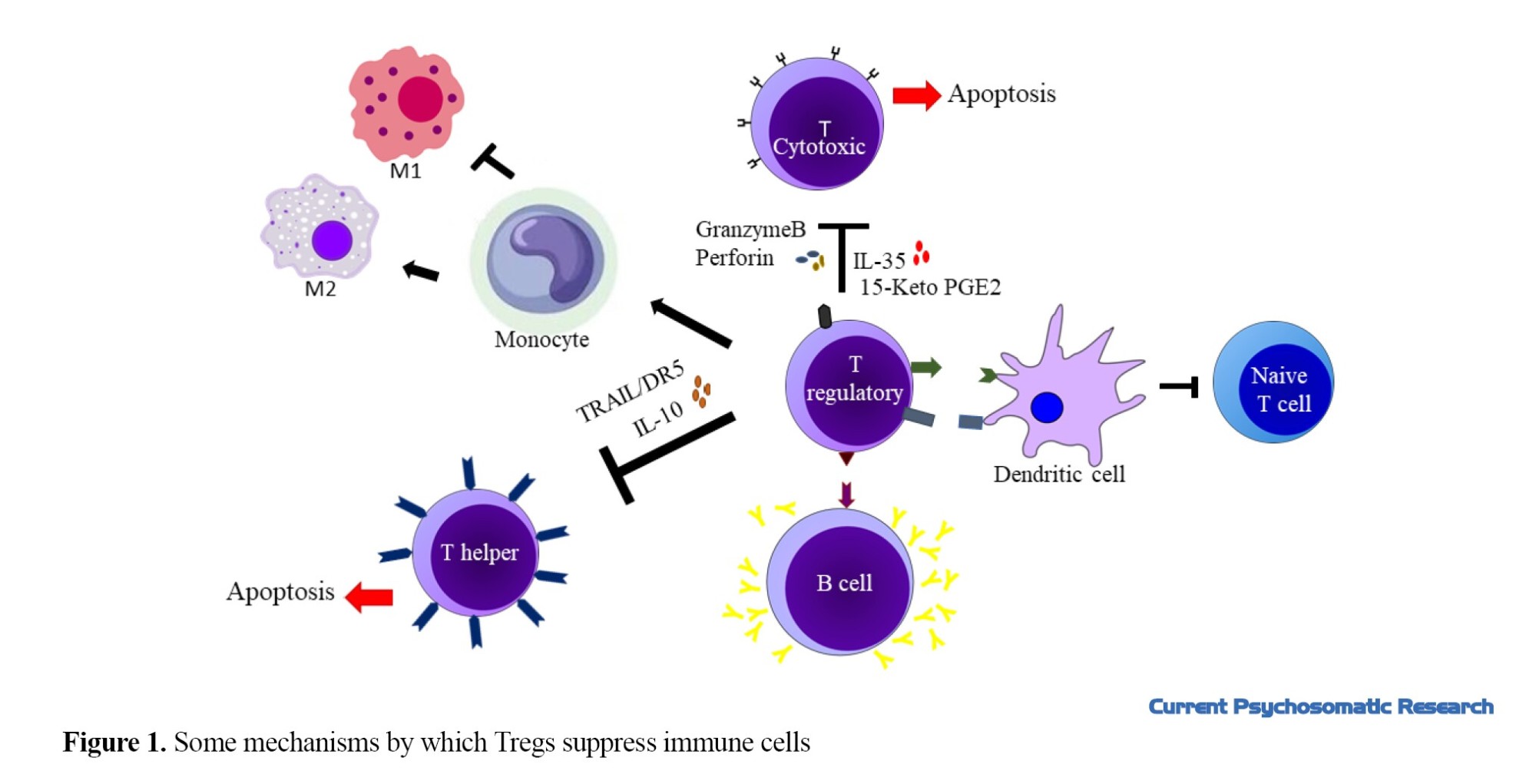
The role of Tregs in neurological disorders
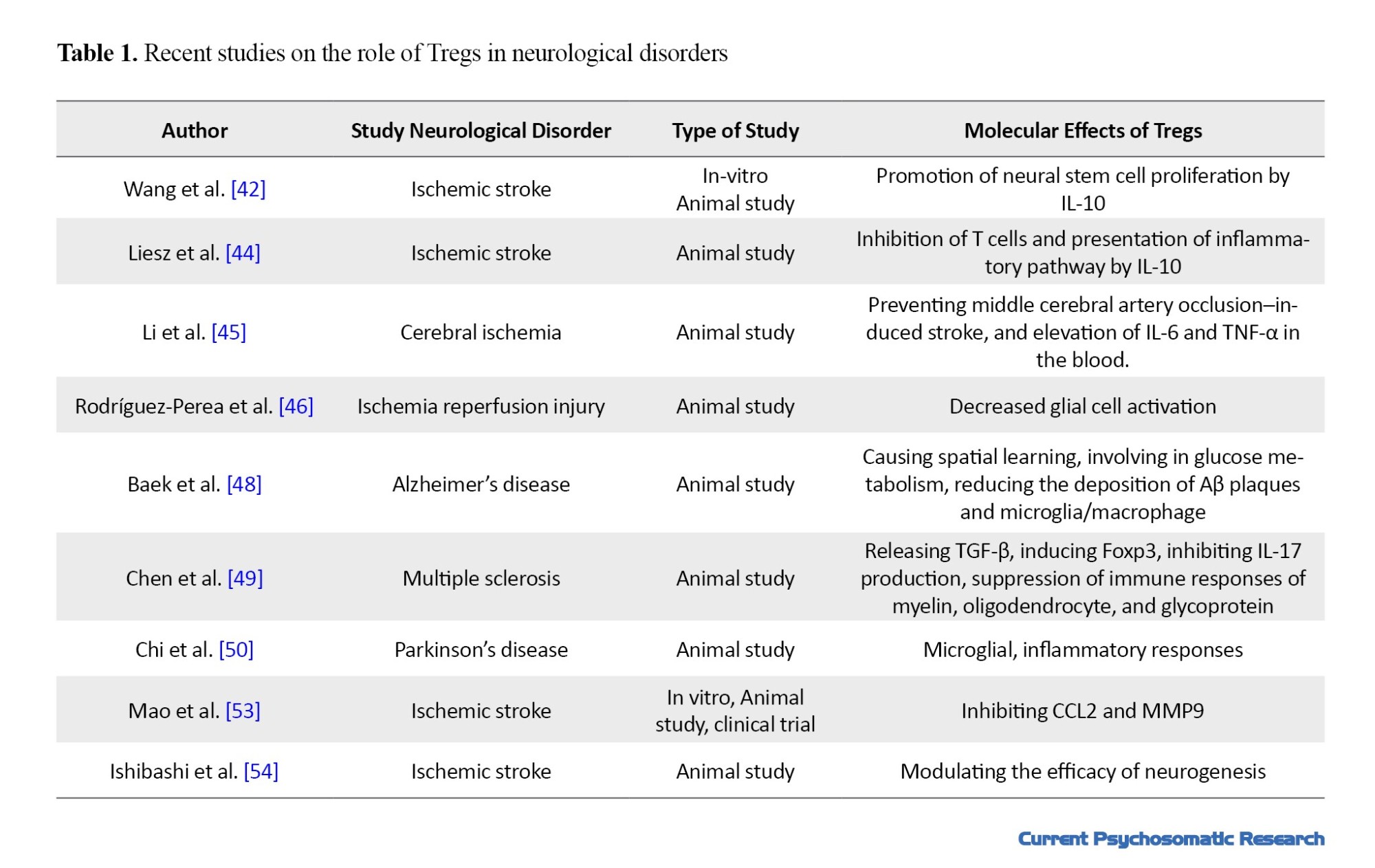
Several studies have demonstrated that Tregs have an essential role in the progress and control of neurological disorders by controlling the cells and their cytokines. Tregs negatively regulate neuroinflammation, enhance neural stem cell proliferation, and reduce brain damage in brain injuries [42-50]. These cells inhibit inflammation resulted from lipopolysaccharide in the prenatal brain tissue [51]. In response to cerebral ischemic injury, Tregs are recruited to the blood-brain barrier (BBB) and exert protection against damage by the expression of PD-L1 [47، 52] and interleukin-1 [48]. They can inhibit brain hemorrhage after stroke by inhibiting neutrophil-derived matrix metalloproteinase-9 (MMP-9) and endothelial-derived CC-chemokine ligand 2 (CCL2) [53، 54]. Furthermore, Tregs express CD39, which causes catalytic inactivation and conversion of extracellular adenosine 5′-triphosphate (ATP), reduces CNS inflammation, and has a role in immune suppression of multiple sclerosis [55]. Recently, using BioNTech mRNA vaccine that codes for disease-related autoantigens optimized for systemic delivery to splenic dendritic cells can activate antigen-specific Tregs and suppress disease-promoting autoreactive T-cells and related cytokines (IL-6, IL-2, IL-17, TNF-α, and IFN-γ). It causes demyelination of the brain and spinal cord [56]. The recent studies on role of Tregs in neurological disorders are summarized in Table 1.
The role of Tregs in ASD
Tregs play a main role in controlling the self-reactivity of the immune system. The deficiency in the number and function of Tregs may impair the immune system and finally intensify the symptoms of ASD [21، 57]. Some studies reported that the transcription of phosphatase and tension homolog (PTEN) genes involved in development of Treg is disrupted in maternal blood of children with ASD and their IL-10 expression decrease. These findings indicate a genetic difference in the maternal blood and reactivity of maternal autoantibodies to fetal brain proteins [58، 59] Several studies showed Th/Treg cell imbalance in ASD patients [57]. Ahmad et al. reported low numbers of Tregs in the peripheral blood of ASD children compared to healthy children. The deficiency rate was 73.3%. Interestingly, these patients have allergic manifestations and a family history of autoimmunity. Giacomo et al. approved that the children with ASD have lower values of Tregs compared to healthy controls. In addition, four out of six patients with severe ASD had a significantly lower frequency of Tregs (35.3%) compared to children with mild or moderate ASD [60]. This evidence can suggest the contributing role of Treg cell deficiency to autoimmunity in the brain [61]. Other studies also showed the number of Tregs in the ASD group was lower in comparison with non-ASD group [62-64]. Tregs inhibit nitrification by stimulating reactions such as microglia α-Synuclein [65]. Therefore, these cells regulate behavioral characteristics of ASD [66].
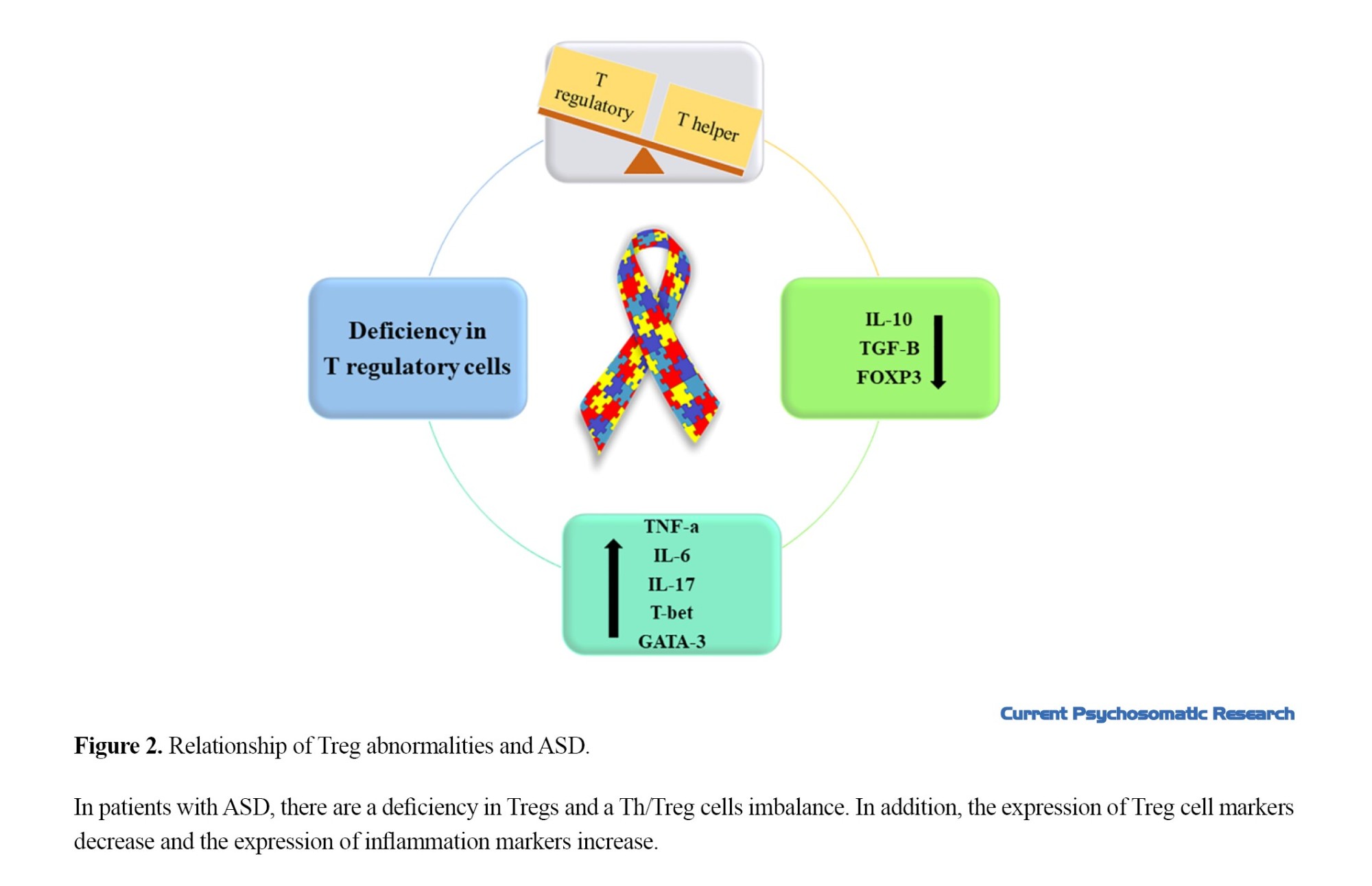
Expression of some Treg cell markers, such as FoxP3 [57، 67، 68], IL-10, and TGF-β were reported to be lower in the peripheral blood of children with ASD [60، 61]. IL-10 induces activation of STAT3 and facilitates the suppression function of Tregs [62، 63]. Ashwood et al. reported that the activities of Treg CD3+IL-10+ decreased in ASD. TGF-β is another cytokine of Tregs. This cytokine has a role in the differentiation and suppression activity of Tregs [61، 62]. Low TGF-β level is has a negative significant association with behavioral test scores [60]. The expression of Treg suppression cytokines such as TNF-α, IL-6, and IL-17, and pro-inflammatory cytokines are higher [13، 15، 67، 69-73].This indicates that Tregs have a role in controlling autistic behavior. These results may be related to clinical evidence of ASD. For example, the severity of gastrointestinal symptoms (diarrhea/constipation) and intestinal permeability in ASD patients is associated with a change in the microbiota composition and frequency of the lymphocytes subtype [74، 75]. Therefore, it is assumed that low Tregs may be associated with microbial dysbiosis. There is a significant increase in the spread of atopic diseases (such as allergies and asthma) among patients with ASD [76، 77]. Tregs are important in maintaining tolerance to several antigens and atopic diseases [78]. Therefore, relative or absolute defects or unstable phenotypes in Tregs may cause atopic diseases in ASD patients [76، 79]. The recent studies that investigated effect of Treg abnormalities on the progression of ASD are summarized in Table 2.
Figure 2 shows the relationship between Treg abnormalities and ASD.
Several studies showed that some drugs and nutritional supplements can help with regulation of the immune system. Bakheet et al. showed that treatment of BTBR mice model of ASD with Resveratrol (a type of natural phenol and a phytoalexin) could have a role in substantial induction of FoxP3+ and reducing T-bet, GATA binding protein 3 (GATA-3), and IL-17A expression in CD4+ cells of mice. They suggested that it may help reduce the complications of ASD [67]. Revealed that combination of Omega-3 and vitamin D had an effect on the main symptoms of ASD. These supplementations may reduce inflammation and increase the number of Tregs and help treat ASD-related symptoms [5، 80-82]. Albekairi et al. demonstrated that the treatment of BTBR mice model of ASD with C-X-C Motif Chemokine receptor 2 (CXCR2) antagonist SB332235 improved the behavior of mice by increasing the Treg-related transcription factors such as IL-10 and Foxp3 [83].
Conclusion
Expression of cytokines in Treg induction decrease in ASD patients which causes Th/Treg cells imbalance and deficiency in Treg cells. This may be clinical evidence of ASD. However, there are scant research on molecular mechanism of Tregs causing healthy behaviors in ASD patients. Future studies on the effect of Th/Treg cells imbalance on attenuating behavior deficits and finding treatments for ASD based on the activation of Tregs and regulation of the immune system are recommended.
Ethical Considerations
Compliance with ethical guidelines
This article is the result of a review study and did not have any human or animal samples. Ethical issues such as avoiding plagiarism, ensuring robustness in collecting relevant data, and publishing rights were considered.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Study design, supervision, and editing: Zahra Yazdani; data collection and preparing the initial draft: Alireza Rafiei; review and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by problems in signs of social interactions and communications and repetitive and restrictive behaviors. These behaviors vary in intensity and time of occurrence [1]. Patients with ASD have feeding problems, such as picky eating or food avoidance. They have difficulty falling or staying asleep and have excessive daytime sleepiness. They have even problems in social functioning such as a deficiency in recognizing emotions and interpreting vocal and visual cues [2، 3]. Their families have high levels of anxiety, stress, and depression, and suffer from high financial burden of the disease [4]. According to the World Health Organization (WHO), 1% of the world’s population suffer ASD [5]. It is a major health problem worldwide. Identifying the involved factors in this disease can help prevent and cure it. The biological mechanisms of ASD are unknown, but the existence of some evidence, such as the association of ASD with congenital rubella infection [6، 7], inflammation [8-10], disruption of cytokine regulation [11-15], and autoantibodies against the proteins of the central nervous system [16-19], confirm the role of the immune system dysfunction in ASD. Several reports have shown an association between ASD and a personal or family history of autoimmune disorders such as psoriasis [17، 18], type 1 diabetes [19-21], celiac disease [22، 23], systemic lupus erythematosus [24، 25], rheumatoid arthritis [26، 27], and autoimmune thyroiditis [28]. Other studies using brain tissues or peripheral blood of people with ASD have also reported a dysfunction in the immune system, including the abundance of active M2 microglial genes in the brain and the genes related to the immune responses [20].
Regulatory T cells (Tregs) play an essential role in regulating the function or maintaining the homeostasis in the immune system. The abnormalities in the population of Tregs can Therefore have a role in progress of ASD. Ellul et al. in a meta-analysis of 13 studies assessed Treg lymphocytes/Th17 lymphocytes imbalance in ASD with the participation of 388 ASD patients and 326 healthy controls [21]. There is no narrative review to investigate the effect of imbalance in the number of Tregs and their role in ASD based on molecular mechanism. Therefore, this review study aims to increase knowledge of the relationships between Treg dysfunction and ASD. We first explain the structure and biological function of Tregs in the immune system and identify its relationship with neurodevelopment, and finally investigate its association with the progression of ASD. The search was carried out in online databases including Google Scholar, Web of Science, PubMed, and Scopus for related studies published up to July 2023.
Physiology and function of Tregs
Tregs are a small group of immune cells that prevent autoimmune diseases and control inflammation by inhibiting autoreactive T cells. Therefore, the development of autoimmune diseases or the lack of control over inflammation in other conditions indicates an insufficient number of Tregs [22].Tregs have different origins. They can originate from the thymus during the development of T cells or from naive CD4+ due to /CD28 stimulation in the presence of cytokines such as transforming growth factor-beta (TGF-β) and interleukin-2 (IL-2) in peripheral blood. Moreover, Tregs have different locations. A group circulates in organs and secondary lymphatic fluids, and the other group resides in non-lymphoid tissues such as colon, fat, and skin, and can play roles such as repairing muscles, differentiating oligodendrocytes, and promoting have remyelination in the brain [22، 24]. Forkhead box P3 (FoxP3) and IL-2 receptor alpha chain (CD25) are two markers of these cells. FoxP3 is a crucial regulator of Treg development and function. The transfer of this marker to naive T cells increases the expression of CD25 and other Treg-related cell surface molecules, cytotoxic T cell-associated antigen-4 (CTLA-4), and glucocorticoid-induced TNF receptor family-related gene/protein (GITR). It suppresses the expression of IL-2, interferon‐gamma (IFN-γ), and interleukin-4. Some studies have shown that high FoxP3 expression can confer suppressive activity to normal non-Treg cells. Other studies have shown that Tregs acquire the properties of effector T cells upon the loss of FoxP3 expression [23، 24]. CD25 is also functionally necessary for Treg development. In vitro studies proved that IL-2 is required for the stable expression of FoxP3 and CD25 in Tregs and increasing their suppressive function [25]. IL-2 also induces the differentiation of Tregs.
Suppression mechanisms of Tregs
Tregs can control immune system by suppression mechanism. These cells interact strongly with dendritic cells and suppress conventional naive T cells [26]. Also, they can regulate CD4+T cells. They can control Th1 response by the expression of miR-146a, and suppression of expression and activation of signal transducer and activator transcription 1 (STAT1) [27]. Also, they can inhibit the proliferation of TH1 cells and the production of their cytokines by expression of interleukin-10 (IL-10) and TGF-β [28-30]. Gut microbiota induces a distinct Treg population that expresses Rorγ, and promotes T helper 17 cell differentiation in colonic TH1/TH17 inflammation [31]. Treg can induce apoptosis in CD4+T cells by expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/death receptor 5 (DR5) [32].These cells can suppress CD8+ T cell proliferation by expression of interleukin-35 [33، 34]. Also, they can upregulate the expression of CD95 and CD95L and express granzyme B and perforin to induce apoptosis in these cells [35-38]. In visceral adipose tissue, Tregs catabolize prostaglandin E2 (PGE2) into the 15-keto PGE2 and suppresses conventional T-cell activation and proliferation [39]. Tregs suppress autoreactive B cells through programmed death ligand 1/programmed cell death protein 1 (PDL1/PD-1) interaction [40]. These cells can convert monocytes to a tolerogenic phenotype (M2 Macrophages) and reduce inflammation [41]. Figure 1 shows some mechanisms by which Tregs control immune cells.

The role of Tregs in neurological disorders
Several studies have demonstrated that Tregs have an essential role in the progress and control of neurological disorders by controlling the cells and their cytokines. Tregs negatively regulate neuroinflammation, enhance neural stem cell proliferation, and reduce brain damage in brain injuries [42-50]. These cells inhibit inflammation resulted from lipopolysaccharide in the prenatal brain tissue [51]. In response to cerebral ischemic injury, Tregs are recruited to the blood-brain barrier (BBB) and exert protection against damage by the expression of PD-L1 [47، 52] and interleukin-1 [48]. They can inhibit brain hemorrhage after stroke by inhibiting neutrophil-derived matrix metalloproteinase-9 (MMP-9) and endothelial-derived CC-chemokine ligand 2 (CCL2) [53، 54]. Furthermore, Tregs express CD39, which causes catalytic inactivation and conversion of extracellular adenosine 5′-triphosphate (ATP), reduces CNS inflammation, and has a role in immune suppression of multiple sclerosis [55]. Recently, using BioNTech mRNA vaccine that codes for disease-related autoantigens optimized for systemic delivery to splenic dendritic cells can activate antigen-specific Tregs and suppress disease-promoting autoreactive T-cells and related cytokines (IL-6, IL-2, IL-17, TNF-α, and IFN-γ). It causes demyelination of the brain and spinal cord [56]. The recent studies on role of Tregs in neurological disorders are summarized in Table 1.

The role of Tregs in ASD
Tregs play a main role in controlling the self-reactivity of the immune system. The deficiency in the number and function of Tregs may impair the immune system and finally intensify the symptoms of ASD [21، 57]. Some studies reported that the transcription of phosphatase and tension homolog (PTEN) genes involved in development of Treg is disrupted in maternal blood of children with ASD and their IL-10 expression decrease. These findings indicate a genetic difference in the maternal blood and reactivity of maternal autoantibodies to fetal brain proteins [58، 59] Several studies showed Th/Treg cell imbalance in ASD patients [57]. Ahmad et al. reported low numbers of Tregs in the peripheral blood of ASD children compared to healthy children. The deficiency rate was 73.3%. Interestingly, these patients have allergic manifestations and a family history of autoimmunity. Giacomo et al. approved that the children with ASD have lower values of Tregs compared to healthy controls. In addition, four out of six patients with severe ASD had a significantly lower frequency of Tregs (35.3%) compared to children with mild or moderate ASD [60]. This evidence can suggest the contributing role of Treg cell deficiency to autoimmunity in the brain [61]. Other studies also showed the number of Tregs in the ASD group was lower in comparison with non-ASD group [62-64]. Tregs inhibit nitrification by stimulating reactions such as microglia α-Synuclein [65]. Therefore, these cells regulate behavioral characteristics of ASD [66].
Expression of some Treg cell markers, such as FoxP3 [57، 67، 68], IL-10, and TGF-β were reported to be lower in the peripheral blood of children with ASD [60، 61]. IL-10 induces activation of STAT3 and facilitates the suppression function of Tregs [62، 63]. Ashwood et al. reported that the activities of Treg CD3+IL-10+ decreased in ASD. TGF-β is another cytokine of Tregs. This cytokine has a role in the differentiation and suppression activity of Tregs [61، 62]. Low TGF-β level is has a negative significant association with behavioral test scores [60]. The expression of Treg suppression cytokines such as TNF-α, IL-6, and IL-17, and pro-inflammatory cytokines are higher [13، 15، 67، 69-73].This indicates that Tregs have a role in controlling autistic behavior. These results may be related to clinical evidence of ASD. For example, the severity of gastrointestinal symptoms (diarrhea/constipation) and intestinal permeability in ASD patients is associated with a change in the microbiota composition and frequency of the lymphocytes subtype [74، 75]. Therefore, it is assumed that low Tregs may be associated with microbial dysbiosis. There is a significant increase in the spread of atopic diseases (such as allergies and asthma) among patients with ASD [76، 77]. Tregs are important in maintaining tolerance to several antigens and atopic diseases [78]. Therefore, relative or absolute defects or unstable phenotypes in Tregs may cause atopic diseases in ASD patients [76، 79]. The recent studies that investigated effect of Treg abnormalities on the progression of ASD are summarized in Table 2.

Figure 2 shows the relationship between Treg abnormalities and ASD.

Several studies showed that some drugs and nutritional supplements can help with regulation of the immune system. Bakheet et al. showed that treatment of BTBR mice model of ASD with Resveratrol (a type of natural phenol and a phytoalexin) could have a role in substantial induction of FoxP3+ and reducing T-bet, GATA binding protein 3 (GATA-3), and IL-17A expression in CD4+ cells of mice. They suggested that it may help reduce the complications of ASD [67]. Revealed that combination of Omega-3 and vitamin D had an effect on the main symptoms of ASD. These supplementations may reduce inflammation and increase the number of Tregs and help treat ASD-related symptoms [5، 80-82]. Albekairi et al. demonstrated that the treatment of BTBR mice model of ASD with C-X-C Motif Chemokine receptor 2 (CXCR2) antagonist SB332235 improved the behavior of mice by increasing the Treg-related transcription factors such as IL-10 and Foxp3 [83].
Conclusion
Expression of cytokines in Treg induction decrease in ASD patients which causes Th/Treg cells imbalance and deficiency in Treg cells. This may be clinical evidence of ASD. However, there are scant research on molecular mechanism of Tregs causing healthy behaviors in ASD patients. Future studies on the effect of Th/Treg cells imbalance on attenuating behavior deficits and finding treatments for ASD based on the activation of Tregs and regulation of the immune system are recommended.
Ethical Considerations
Compliance with ethical guidelines
This article is the result of a review study and did not have any human or animal samples. Ethical issues such as avoiding plagiarism, ensuring robustness in collecting relevant data, and publishing rights were considered.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Study design, supervision, and editing: Zahra Yazdani; data collection and preparing the initial draft: Alireza Rafiei; review and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Hiremath CS, Sagar KJV, Yamini BK, Girimaji AS, Kumar R, Sravanti SL, et al. Emerging behavioral and neuroimaging biomarkers for early and accurate characterization of autism spectrum disorders: A systematic review. Transl Psychiatry. 2021; 11(1):42. [DOI:10.1038/s41398-020-01178-6] [PMID]
- Baraskewich J, von Ranson KM, McCrimmon A, McMorris CA. Feeding and eating problems in children and adolescents with autism: A scoping review. Autism. 2021; 25(6):1505-19.[DOI:10.1177/1362361321995631] [PMID] [PMCID]
- Whelan S, Mannion A, Madden A, Berger F, Costello R, Ghadiri S, et al. Examining the relationship between sleep quality, social functioning, and behavior problems in children with autism spectrum disorder: A systematic review. Nat Sci Sleep. 2022; 14:675-695. [DOI:10.2147/NSS.S239622] [PMID] [PMCID]
- Ponson L, Gomot M, Blanc R, Barthelemy C, Roux S, Munnich A, et al. 22q13 deletion syndrome: Communication disorder or autism? Evidence from a specific clinical and neurophysiological phenotype. Transl Psychiatry. 2018; 8(1):146. [DOI:10.1038/s41398-018-0212-9] [PMID] [PMCID]
- Infante M, Sears B, Rizzo AM, Mariani Cerati D, Caprio M, Ricordi C, et al. Omega-3 PUFAs and vitamin D co-supplementation as a safe-effective therapeutic approach for core symptoms of autism spectrum disorder: Case report and literature review. Nutr Neurosci. 2020; 23(10):779-90 [DOI:10.1080/1028415X.2018.1557385] [PMID]
- Chess S. Follow-up report on autism in congenital rubella. J Autism Child Schizophr. 1977; 7(1):69-81. [DOI:10.1007/BF01531116] [PMID]
- Berger BE, Navar-Boggan AM, Omer SB. Congenital rubella syndrome and autism spectrum disorder prevented by rubella vaccination--United States, 2001-2010. BMC Public Health. 2011; 11:340. [DOI:10.1186/1471-2458-11-340] [PMID] [PMCID]
- Smedler E, Kleppe J, Neufeld J, Lundin K, Bölte S, Landén M. Cerebrospinal fluid and serum protein markers in autism: A co-twin study. J Neurochem. 2021; 158(3):798-806. [DOI:10.1111/jnc.15338] [PMID]
- Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002; 45(1):1-6. [DOI:10.1159/000048665] [PMID]
- Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology. 2017; 42(1):284-98. [DOI:10.1038/npp.2016.158] [PMID] [PMCID]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011; 25(1):40-5. [DOI:10.1016/j.bbi.2010.08.003] [PMID] [PMCID]
- Xie J, Huang L, Li X, Li H, Zhou Y, Zhu H, et al. Immunological cytokine profiling identifies TNF-α as a key molecule dysregulated in autistic children. Oncotarget. 2017; 8(47):82390-8. [DOI:10.18632/oncotarget.19326] [PMID] [PMCID]
- Eftekharian MM, Ghafouri-Fard S, Noroozi R, Omrani MD, Arsang-Jang S, Ganji M, et al. Cytokine profile in autistic patients. Cytokine. 2018; 108:120-6. [DOI:10.1016/j.cyto.2018.03.034] [PMID]
- Al-Ayadhi LY. Pro-inflammatory cytokines in autistic children in central Saudi Arabia. Neurosciences. 2005; 10(2):155-8. [PMID]
- Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. Plos One. 2011; 6(5):e20470. [DOI:10.1371/journal.pone.0020470] [PMID] [PMCID]
- Singh VK, Lin SX, Newell E, Nelson C. Abnormal measles-mumps-rubella antibodies and CNS autoimmunity in children with autism. J Biomed Sci. 2002; 9(4):359-64. [DOI:10.1007/BF02256592] [PMID]
- Libbey JE, Coon HH, Kirkman NJ, Sweeten TL, Miller JN, Stevenson EK, et al. Are there enhanced MBP autoantibodies in autism? J Autism Dev Disord. 2008; 38(2):324-32. [DOI:10.1007/s10803-007-0400-6] [PMID]
- Wills S, Rossi CC, Bennett J, Martinez-Cerdeño V, Ashwood P, Amaral DG, et al. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Mol Autism. 2011; 2:5. [DOI:10.1186/2040-2392-2-5] [PMID] [PMCID]
- Mostafa GA, Al-Ayadhi LY. The relationship between the increased frequency of serum antineuronal antibodies and the severity of autism in children. Eur J Paediatr Neurol. 2012; 16(5):464-8. [DOI:10.1016/j.ejpn.2011.12.010] [PMID]
- Brown AS, Surcel HM, Hinkka-Yli-Salomäki S, Cheslack-Postava K, Bao Y, Sourander A. Maternal thyroid autoantibody and elevated risk of autism in a national birth cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2015; 57:86-92. [DOI:10.1016/j.pnpbp.2014.10.010] [PMID] [PMCID]
- Ellul P, Rosenzwajg M, Peyre H, Fourcade G, Mariotti-Ferrandiz E, Trebossen V, et al. Regulatory T lymphocytes/Th17 lymphocytes imbalance in autism spectrum disorders: Evidence from a meta-analysis. Mol Autism. 2021; 12(1):68. [DOI:10.1186/s13229-021-00472-4] [PMID] [PMCID]
- Roncarolo MG, Levings MK. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol. 2000; 12(6):676-83. [DOI:10.1016/S0952-7915(00)00162-X] [PMID]
- Kessel A, Haj T, Peri R, Snir A, Melamed D, Sabo E, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 2012; 11(9):670-7. [DOI:10.1016/j.autrev.2011.11.018] [PMID]
- Janson PC, Winerdal ME, Marits P, Thörn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. Plos One. 2008; 3(2):e1612.[DOI:10.1371/journal.pone.0001612] [PMID] [PMCID]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005; 6(11):1142-51. [DOI:10.1038/ni1263] [PMID]
- Akkaya B, Oya Y, Akkaya M, Al Souz J, Holstein AH, Kamenyeva O, et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol. 2019; 20(2):218-31. [DOI:10.1038/s41590-018-0280-2] [PMID] [PMCID]
- Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010; 142(6):914-29. [DOI:10.1016/j.cell.2010.08.012] [PMID] [PMCID]
- Bell E. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. Nat Rev Immunol. 2001; 1:4. [Link]
- Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996; 183(6):2669-74. [DOI:10.1084/jem.183.6.2669] [PMID] [PMCID]
- Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001; 166(6):3789-96. [DOI:10.4049/jimmunol.166.6.3789] [PMID]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, et al. Mucosal immunology. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science. 2015; 349(6251):993-7. [DOI:10.1126/science.aaa9420] [PMID] [PMCID]
- Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4(+)CD25(+) regulatory T cells. Cell Death Differ. 2007; 14(12):2076-84. [DOI:10.1038/sj.cdd.4402220] [PMID]
- Eslami M, Rafiei A, Baghbanian SM, Fattahi S, Yazdani Z, Valadan R, et al. Serum levels and genetic variation of IL-35 are associated with multiple sclerosis: A population-based case-control study. Immunol Res. 2022; 70(1):75-85. [DOI:10.1007/s12026-021-09246-9] [PMID]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007; 450(7169):566-9. [DOI:10.1038/nature06306] [PMID]
- Strauss L, Bergmann C, Whiteside TL. Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol. 2009; 182(3):1469-80. [DOI:10.4049/jimmunol.182.3.1469] [PMID] [PMCID]
- Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007; 27(4):635-46. [DOI:10.1016/j.immuni.2007.08.014] [PMID]
- Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: Contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005; 174(4):1783-6. [DOI:10.4049/jimmunol.174.4.1783] [PMID]
- Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004; 21(4):589-601. [DOI:10.1016/j.immuni.2004.09.002] [PMID]
- Schmidleithner L, Thabet Y, Schönfeld E, Köhne M, Sommer D, Abdullah Z, et al. Enzymatic activity of HPGD in treg cells suppresses tconv cells to maintain adipose tissue homeostasis and prevent metabolic dysfunction. Immunity. 2019; 50(5):1232-48.e14. [DOI:10.1016/j.immuni.2019.03.014] [PMID]
- Gotot J, Gottschalk C, Leopold S, Knolle PA, Yagita H, Kurts C, et al. Regulatory T cells use programmed death 1 ligands to directly suppress autoreactive B cells in vivo. Proc Natl Acad Sci U S A. 2012; 109(26):10468-73. [DOI:10.1073/pnas.1201131109] [PMID] [PMCID]
- Romano M, Fanelli G, Tan N, Nova-Lamperti E, McGregor R, Lechler RI, et al. Expanded regulatory T cells induce alternatively activated monocytes with a reduced capacity to expand T helper-17 cells. Front Immunol. 2018; 9:1625. [DOI:10.3389/fimmu.2018.01625] [PMID] [PMCID]
- Wang J, Xie L, Yang C, Ren C, Zhou K, Wang B, et al. Activated regulatory T cell regulates neural stem cell proliferation in the subventricular zone of normal and ischemic mouse brain through interleukin 10. Front Cell Neurosci. 2015; 9:361. [DOI:10.3389/fncel.2015.00361] [PMID] [PMCID]
- Lee HT, Liu SP, Lin CH, Lee SW, Hsu CY, Sytwu HK, et al. A crucial role of CXCL14 for promoting regulatory T cells activation in stroke. Theranostics. 2017; 7(4):855-75. [DOI:10.7150/thno.17558] [PMID] [PMCID]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009; 15(2):192-9. [DOI:10.1038/nm.1927] [PMID]
- Li P, Mao L, Zhou G, Leak RK, Sun BL, Chen J, et al. Adoptive regulatory T-cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke. 2013; 44(12):3509-15. [DOI:10.1161/STROKEAHA.113.002637] [PMID] [PMCID]
- Rodríguez-Perea AL, Gutierrez-Vargas J, Cardona-Gómez GP, Guarin CJM, Rojas M, Hernández PAV. Atorvastatin modulates regulatory T cells and attenuates cerebral damage in a model of transient middle cerebral artery occlusion in rats. J Neuroimmune Pharmacol. 2017; 12(1):152-62. [DOI:10.1007/s11481-016-9706-5]
- Li P, Wang L, Zhou Y, Gan Y, Zhu W, Xia Y, et al. C-C Chemokine receptor type 5 (CCR5)-mediated docking of transferred tregs protects against early blood-brain barrier disruption after stroke. J Am Heart Assoc. 2017; 6(8):e006387. [DOI:10.1161/JAHA.117.006387] [PMID] [PMCID]
- Baek H, Ye M, Kang GH, Lee C, Lee G, Choi DB, et al. Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg-AD Alzheimer's disease model. Oncotarget. 2016; 7(43):69347-57. [DOI:10.18632/oncotarget.12469] [PMID] [PMCID]
- Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008; 180(11):7327-37. [DOI:10.4049/jimmunol.180.11.7327] [PMID] [PMCID]
- Chi Y, Fan Y, He L, Liu W, Wen X, Zhou S, et al. Novel role of aquaporin-4 in CD4+ CD25+ T regulatory cell development and severity of Parkinson's disease. Aging Cell. 2011; 10(3):368-82. [DOI:10.1111/j.1474-9726.2011.00677.x] [PMID]
- Wang F, Xiao M, Chen RJ, Lin XJ, Siddiq M, Liu L. Adoptive transfer of T regulatory cells inhibits lipopolysaccharide-induced inflammation in fetal brain tissue in a late-pregnancy preterm birth mouse model. Cell Biol Int. 2017; 41(2):155-62. [DOI:10.1002/cbin.10710] [PMID]
- Mracsko E, Liesz A, Stojanovic A, Lou WP, Osswald M, Zhou W, et al. Antigen dependently activated cluster of differentiation 8-positive T cells cause perforin-mediated neurotoxicity in experimental stroke. J Neurosci. 2014; 34(50):16784-95. [DOI:10.1523/JNEUROSCI.1867-14.2014] [PMID] [PMCID]
- Mao L, Li P, Zhu W, Cai W, Liu Z, Wang Y, et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain. 2017; 140(7):1914-31. [DOI:10.1093/brain/awx111] [PMID] [PMCID]
- Ishibashi S, Maric D, Mou Y, Ohtani R, Ruetzler C, Hallenbeck JM. Mucosal tolerance to E-selectin promotes the survival of newly generated neuroblasts via regulatory T-cell induction after stroke in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2009; 29(3):606-20. [DOI:10.1038/jcbfm.2008.153] [PMID] [PMCID]
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood. 2007; 110(4):1225-32. [DOI:10.1182/blood-2006-12-064527] [PMID]
- Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021; 371(6525):145-53. [DOI:10.1126/science.aay3638] [PMID]
- Ahmad SF, Zoheir KMA, Ansari MA, Nadeem A, Bakheet SA, Al-Ayadhi LY, et al. Dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in children with autism. Mol Neurobiol. 2017; 54(6):4390-400. [DOI:10.1007/s12035-016-9977-0] [PMID]
- Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB. Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Transl Psychiatry. 2011; 1(10):e48. [DOI:10.1038/tp.2011.48] [PMID] [PMCID]
- Robinson-Agramonte MLA, Noris García E, Fraga Guerra J, Vega Hurtado Y, Antonucci N, Semprún-Hernández N, et al. Immune dysregulation in autism spectrum disorder: What do we know about it? Int J Mol Sci. 2022; 23(6):3033. [DOI:10.3390/ijms23063033] [PMID] [PMCID]
- De Giacomo A, Gargano CD, Simone M, Petruzzelli MG, Pedaci C, Giambersio D, et al. B and T immunoregulation: A new insight of B regulatory lymphocytes in autism spectrum disorder. Front Neurosci. 2021; 15:732611. [DOI:10.3389/fnins.2021.732611] [PMID] [PMCID]
- Mostafa GA, Al Shehab A, Fouad NR. Frequency of CD4+CD25high regulatory T cells in the peripheral blood of Egyptian children with autism. J Child Neurol. 2010; 25(3):328-35. [DOI:10.1177/0883073809339393] [PMID]
- Cao W, Luo C, Fan Z, Lei M, Cheng X, Shi Z, et al. Analysis of potential biomarkers and immune infiltration in autism based on bioinformatics analysis. Medicine. 2023; 102(19):e33340. [DOI:10.1097/MD.0000000000033340] [PMID] [PMCID]
- Uddin MN, Manley K, Lawrence DA. Altered meningeal immunity contributing to the autism-like behavior of BTBR T+Itpr3tf /J mice. Brain Behav Immun Health. 2022; 26:100563. [DOI:10.1016/j.bbih.2022.100563] [PMID] [PMCID]
- Nie ZQ, Han D, Zhang K, Li M, Kwon HK, Im SH, et al. TH1/Treg ratio may be a marker of autism in children with immune dysfunction. Res Autism Spectr Disord. 2023; 101:102085. [DOI:10.1016/j.rasd.2022.102085]
- Moaaz M, Youssry S, Elfatatry A, El Rahman MA. Th17/Treg cells imbalance and their related cytokines (IL-17, IL-10 and TGF-β) in children with autism spectrum disorder. J Neuroimmunol. 2019; 337:577071. [DOI:10.1016/j.jneuroim.2019.577071] [PMID]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE. Nitrated {alpha}-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J Immunol. 2009; 182(7):4137-49. [DOI:10.4049/jimmunol.0803982] [PMID] [PMCID]
- Bakheet SA, Alzahrani MZ, Ansari MA, Nadeem A, Zoheir KMA, Attia SM, et al. Resveratrol ameliorates dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in a BTBR T + tf/J mouse model of autism. Mol Neurobiol. 2017; 54(7):5201-12. [DOI:10.1007/s12035-016-0066-1] [PMID]
- Akbari M, Eghtedarian R, Hussen BM, Eslami S, Taheri M, Neishabouri SM, et al. Assessment of expression of regulatory T cell differentiation genes in autism spectrum disorder. Front Mol Neurosci. 2022; 15:939224. [DOI:10.3389/fnmol.2022.939224] [PMID] [PMCID]
- Basheer S, Venkataswamy MM, Christopher R, Van Amelsvoort T, Srinath S, Girimaji SC, et al. Immune aberrations in children with Autism Spectrum Disorder: A case-control study from a tertiary care neuropsychiatric hospital in India. Psychoneuroendocrinology. 2018; 94:162-7. [DOI:10.1016/j.psyneuen.2018.05.002] [PMID]
- Al-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. J Neuroinflammation. 2012; 9:158. [DOI:10.1186/1742-2094-9-158] [PMID] [PMCID]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016; 351(6276):933-9. [DOI:10.1126/science.aad0314] [PMID] [PMCID]
- Saghazadeh A, Ataeinia B, Keynejad K, Abdolalizadeh A, Hirbod-Mobarakeh A, Rezaei N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J Psychiatr Res. 2019; 115:90-102. [DOI:10.1016/j.jpsychires.2019.05.019] [PMID]
- Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: Mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004; 24(6):664-73. [DOI:10.1007/s10875-004-6241-6] [PMID]
- Bjørklund G, Pivina L, Dadar M, Meguid NA, Semenova Y, Anwar M, et al. Gastrointestinal alterations in autism spectrum disorder: What do we know? Neurosci Biobehav Rev. 2020; 118:111-20. [DOI:10.1016/j.neubiorev.2020.06.033] [PMID]
- Rose DR, Yang H, Careaga M, Angkustsiri K, Van de Water J, Ashwood P. T cell populations in children with autism spectrum disorder and co-morbid gastrointestinal symptoms. Brain Behav Immun Health. 2020; 2:100042. [DOI:10.1016/j.bbih.2020.100042] [PMID] [PMCID]
- Dai YX, Tai YH, Chang YT, Chen TJ, Chen MH. Increased risk of atopic diseases in the siblings of patients with autism spectrum disorder: A nationwide population-based cohort study. J Autism Dev Disord. 2019; 49(11):4626-33. [DOI:10.1007/s10803-019-04184-w] [PMID]
- Walker JA, McKenzie ANJ. TH2 cell development and function. Nat Rev Immunol. 2018; 18(2):121-33. [DOI:10.1038/nri.2017.118] [PMID]
- Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of treg in immune regulation of allergic diseases. Eur J Immunol. 2010; 40(5):1232-40. [DOI:10.1002/eji.200940045] [PMID]
- Lan F, Zhang N, Bachert C, Zhang L. Stability of regulatory T cells in T helper 2-biased allergic airway diseases. Allergy. 2020; 75(8):1918-26. [DOI:10.1111/all.14257] [PMID]
- Peterle L, Sanfilippo S, Tonacci A, Li Pomi F, Borgia F, Gangemi S. Common pathogenetic traits of atopic dermatitis and autism spectrum disorders, potential connections and treatments: trivial Th2 inflammation or much more? Front Immunol. 2023; 14:1201989. [DOI:10.3389/fimmu.2023.1201989] [PMID] [PMCID]
- Gutiérrez S, Svahn SL, Johansson ME. Effects of omega-3 fatty acids on immune cells. Int J Mol Sci. 2019; 20(20):5028. [DOI:10.3390/ijms20205028] [PMID] [PMCID]
- Mostafa GA, Al-Ayadhi LY. Reduced serum concentrations of 25-hydroxy vitamin D in children with autism: Relation to autoimmunity. J Neuroinflammation. 2012; 9:201. [DOI:10.1186/1742-2094-9-201] [PMID] [PMCID]
- Albekairi NA, Nadeem A, Ansari MA, Attia SM, Bakheet SA, Alanazi MM, et al. CXCR2 antagonist SB332235 mitigates deficits in social behavior and dysregulation of Th1/Th22 and T regulatory cell-related transcription factor signaling in male BTBR T+ Itpr3tf/J mouse model of autism. Pharmacol Biochem Behav. 2022; 217:173408. [DOI:10.1016/j.pbb.2022.173408] [PMID]
Type of Study: review |
Subject:
Psychology of Exceptional Children
Received: 2023/03/20 | Accepted: 2023/05/25 | Published: 2023/07/1
Received: 2023/03/20 | Accepted: 2023/05/25 | Published: 2023/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







